The cell cycle is an orderly series of events that take place in a cell leading to duplication of its DNA and division of cytoplasm & organelles to produce two daughter cells. In other words, the cellular events are a sequential expression of different genes. Regulation of the cell cycle is an extremely important step as it o
To read more on cell cycle, click on this link
Before we proceed further, I want to make sure that you clearly know the difference between cell cycle regulation and cell cycle checkpoints.
What is cell cycle regulation?
Cell cycle regulation is an internal process to control the rate of cell growth and division. Cell cycle regulation is a necessary process because, without cell cycle regulation, cells could grow in an uncontrolled manner and cause great problems for the host organism. An example of uncontrolled cell division is seen in cancerous cells and we all know how dangerous they are.
What are Cell Cycle Checkpoints?
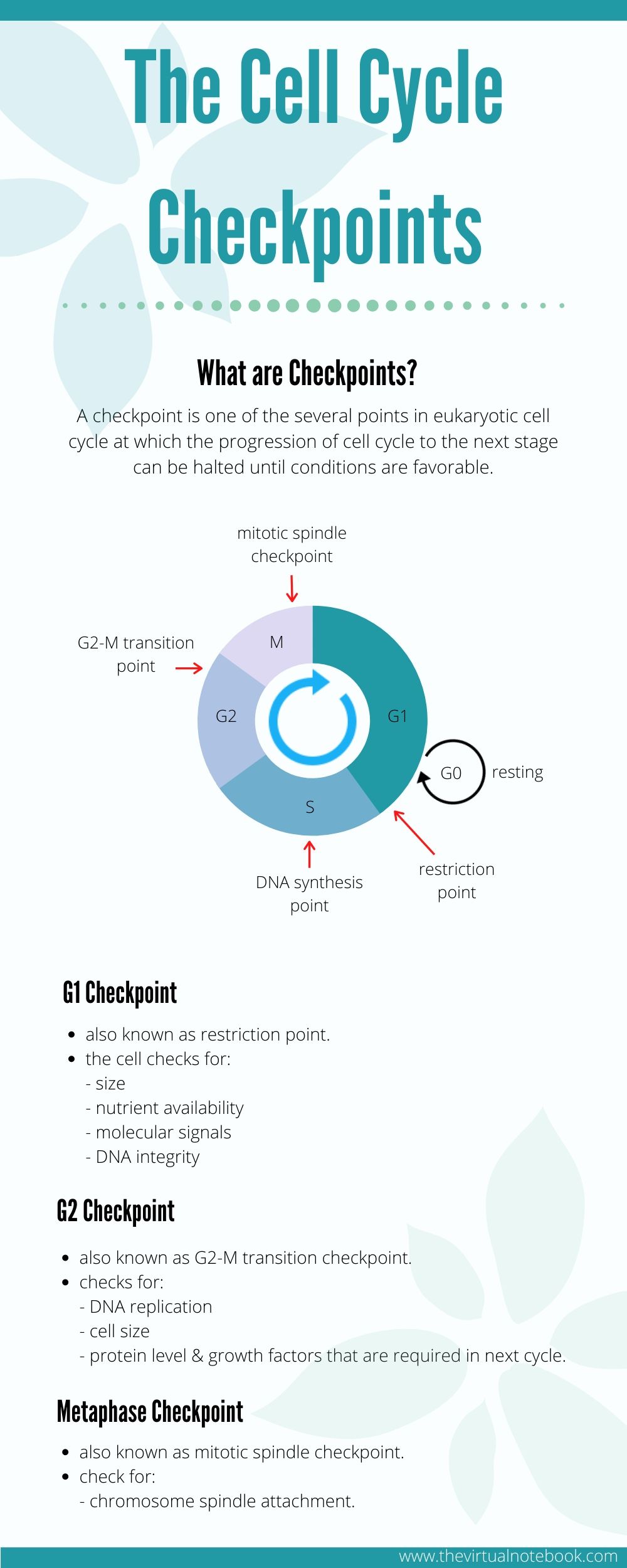
A checkpoint is one of several points in the eukaryotic cell cycle at which the progression of a cell to the next stage in the cell cycle can be halted until conditions are favorable. In multiple cell division, it is most important that each new daughter cell must receive a complete and accurate copy of the genome. Moreover, chromosomes must be duplicated once and only once before mitosis
In all these situations, the order is maintained by checkpoints. Checkpoints ensure that each of the various events that make up the cell cycle occurs accurately and in the proper order. Also, cells continually monitor DNA damage occurring throughout the division cycle. In other words, checkpoints represent an elegant solution to the problem of ordering DNA synthesis and cell division.
I will tell more about checkpoints later in this post… So, stay connected…
Regulation of cell cycle
As I told earlier, the cell cycle regulation is a necessary process because, without cell cycle regulation, cells could grow in an uncontrolled manner and causing great problems for the host organism.
During the cell cycle, some proteins become active and/or synthesized at certain times in the cell cycle. For example, enzymes involved in DNA synthesis are commonly induced in G1 and S phase. Similarly, tubulin for the spindle is made during G2.
The time required to complete certain events varies greatly from one cell type to another, even in the same organism. A typical human cell might
The timing of events in the cell cycle is controlled by mechanisms that are both internal (positive regulation) and external (negative regulation)to the cell. Moreover, the cell cycle is controlled by regulator molecules that either promote the process or stop it from progressing.
Internal mitotic inducer (positive regulation)
Internal factors include protein or enzymes present within the cell which comes into play during the cell cycle. The cytoplasm of the mitotic cell contains diffusible factors that could induce mitosis. An example of diffusible protein is MPF.
Maturation Promoting Factor (MPF)
The entry of a cell into M phase is initiated by a protein called maturation‐promoting factor ( MPF). It consists of two subunits:
- larger subunit
- smaller subunit
Larger subunit (p45) has a
Smaller subunit (p32) has a molecular weight of 32,000. It is a regulatory subunit called cyclin.
Cellular targets of MPF
While the cellular targets of MPF are not yet known, three are most relevant to mitosis:
- Microtubules are phosphorylated, and this is essential to their transition from interphase stability to metaphase instability.
- Histone H1 is phosphorylated and this leads to chromosome condensation.
- Nuclear lamins are phosphorylated, and this leads to dis-aggregation of the nuclear envelope.
The activity of MPF fluctuates throughout the cell cycle. It is most active at the end of G2 while falls rapidly after the completion of mitosis.
Cyclin
Cyclin is an activator of MPF kinase. As interphase proceeds from S and G2, a cyclin produces and accumulates in the cell until there is enough of it to bind to MPF. The term cyclin was coined because the concentration of this regulatory protein rises and falls in a predictable pattern with each cell cycle. When the cyclin concentration rises, the kinase becomes active, causing the cell to enter M phase. The degradation of cyclin essentially completes the mitotic induction cycle.
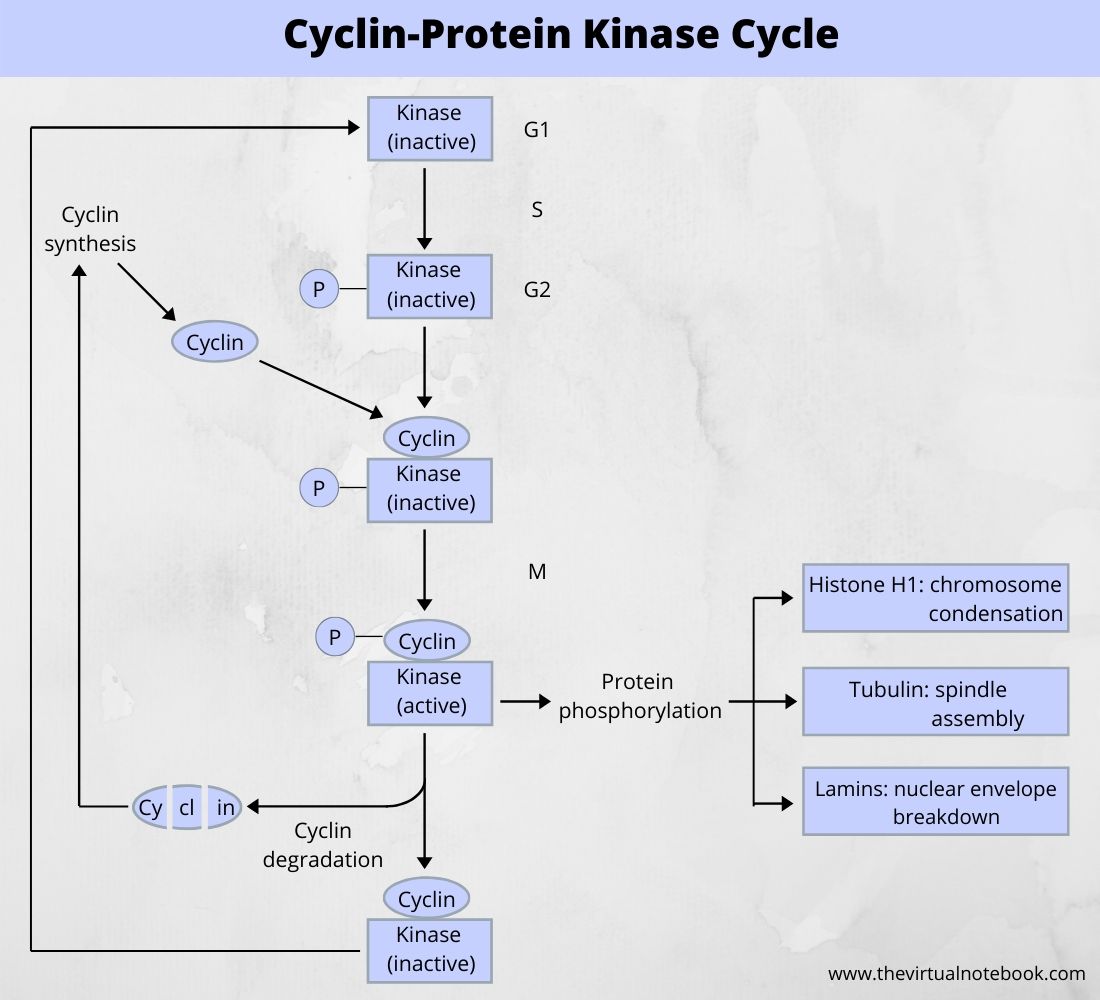
The cyclin-protein cycle regulates the entry of the cell into M-phase from G2. The kinase protein is always present but it comes into play when it binds to cyclin at the end of G2. Cyclin accumulates during G1 and S and reaches a peak at G2. After M-phase, it will break down. The phosphorylated kinase is inactive while dephosphorylated kinase is active if suitably bound to cyclin. The kinase is also called maturation-promoting-factor (MPF) or p34.
To summarize, the progression of cells into mitosis depends on an enzyme whose sole activity is to phosphorylate other proteins. The activity of this enzyme is control by a subunit whose concentration varies from one stage of the cell cycle to another.
External mitotic inducers (negative regulation)
In addition to internal control, the initiation of cell cycle events in higher eukaryotes is affected by external factors, such as hormones. For example, when a wound heals, cells divide to accomplish the process of healing. They stop further cell division when the wound heals. In such a case,
Examples of external factors:
- transforming growth factors (TGF): TGF is a protein (chalones) and produces by epithelial tissues. It can inhibit cell division in some epithelial-derived
tumors . - erythropoietin (EPO): it is produce by liver and kidney and encourages the production of erythrocytes in the bone marrow. A typical red blood cell has a lifespan of about 120 days in humans. If their number decreases or oxygen level is low in blood, EPO is made and released in increased amounts.
- platelet-derived growth factor (PDGF): blood platelets make this protein in smooth muscle cells and endothelial cells that are present in blood vessel walls and lining of blood vessel lumen respectively. These cells produce PGDF in a blood vessel injury. It stimulates the division of smooth muscle cells and fibroblasts, which heal the wound.
There are so many other examples of such hormones like fibroblast growth factor, nerve growth factor, epidermal growth factor, etc. However, the mechanism by which hormones and growth factors stimulate cell cycle is not clear.
Most of the growth factors for animal cells are polypeptide, which binds to specific receptors at the cell membrane. These receptors often connected to the tyrosine-protein kinase. Following binding, the factor-receptor complex aggregate and internalized by coated vesicles. At last, the receptor will recycle back to the cell surface.
Checkpoints
Checkpoints halt the progress of the cell cycle if any of the chromosomal DNA is damaged or certain critical processes have not been properly completed. Many of the proteins of the checkpoint machinery have no role in normal cell cycle events and are only call into action when an abnormality occurs.
Checkpoints
If the DNA damage is beyond repair, the checkpoint mechanism can transmit a signal that leads either to
- the death of the cell (apoptosis) or
- its conversion to a state of permanent cell cycle arrest.
Various checkpoints
There are three major checkpoints in the cell cycle and these are as follows:
- G1 checkpoint (Start or restriction checkpoint or Major Checkpoint)
- G2/M checkpoint
- metaphase checkpoint (spindle checkpoint)
G1 Checkpoint
It is a restriction point in mammalian cells and the start point in yeast. It is the main decision point for a cell i.e., the primary point at which it must choose whether to divide or not. As the cell continues through G1, it can either delay G1, enter G0, or proceed past the restriction point. DNA damage is the main indication for a cell to “restrict” and not enter the cell cycle.
At the G1 checkpoint, a cell checks whether internal and external conditions are right for the division. For example, size, nutrients availability, various molecular signals, DNA integrity, etc.:
If a cell doesn’t get the go-ahead cues it needs at the G1 checkpoint, it may leave the cell cycle and enter a resting state called G0 phase. Some cells stay permanently in G0, while others resume dividing if conditions improve.
G2 checkpoint
Following DNA replication in S phase, the cell undergoes a growth phase known as G2. Multiple mechanistic checkpoints may involve in this transition from G2 to M.
Similar to S Phase, G2 experiences a DNA damage checkpoint. The cell is once more examined for sites of DNA damage or incomplete replication
If the cell detects any error or damage, the cell will pause at the G2 checkpoint to allow for repairs. If the checkpoint mechanisms detect problems with the DNA, the cell cycle will halt, and the cell attempts to either complete DNA replication or repair the damaged DNA. Apart from this, G2 checkpoints monitors the level of proteins and growth factors that are essential in the next phase of the cell cycle
In the case of irreparable damage, the cell may undergo apoptosis or programmed cell death to ensure that any of the damaged DNA will not pass to the daughter cells.
M- phase checkpoint (spindle checkpoint)
The mitotic spindle checkpoint occurs at the point in metaphase where all the chromosomes should/have aligned at the mitotic plate and be under bipolar tension.
Here, the cell examines whether all the sister chromatids
If the chromosomes are not attached to the spindle apparatus, M- checkpoint will stop further cell cycle
Sources and links
Karp’s cell and molecular biology: concepts and experiments 8th edition by Janet Iwasa and Wallace Marshall, chapter 14.
Molecular Biology of the cell, 5th edition, Bruce Alberts, Alexander Johnson, Julian Lewis, Martin Raff, Keith Roberts, and Peter Walter, chapter no. 17.
The cell: A molecular approach by Geoffrey M Cooper, chapter 16, the cell cycle.
Philipp Kaldis(Ed.) Cell Cycle Regulation
Cell and Molecular Biology by Prakash S. Lohar, page no. 204-205
https://www.wisegeek.com/what-is-cell-cycle-regulation.htm
https://www.onlinebiologynotes.com/checkpoints-and-regulation-of-cell-cycle%ef%bb%bf/
https://www.easybiologyclass.com/cell-cycle-checkpoints-regulation-cancer/