In this article, we will learn about T cell development and its differentiation pathway, including the selection events, which decides their fate. But first, let’s begin with the role of these T cells in our immunity.
Function of T cells
T cells are a part of the immune system that plays a central role in the adaptive immune response. In addition, each of the several million T cells circulating in the body expresses a distinct T cell receptor. It’s these receptors that help initiate the immune response by interacting with the MHC/peptide complexes on antigen-presenting cells.
T cell receptor
The T cell receptor (TCR) is a protein complex that is found on the surface of T cells or T lymphocytes. It is responsible for recognizing fragments of antigens as peptides bind to major histocompatibility complex (MHC) molecules. It does that in such a way, that it allows the T cells to identify which cells are expressing pathogenic epitopes within their MHC molecules.
T cell receptors (TCRs) are of two types: αβ and γδ. In humans, in 95% of T cells, the TCR consists of an α chain and a β chain, whereas in 5% of T cells, the TCR consists of γ and δ chains. This ratio changes during ontogeny and in diseased states (such as leukaemia). It also differs between species.
The αβ TCRs recognize peptide fragments presented by class I or class II molecules of the MHC. Whereas, the γδ TCRs may recognize free peptides. Also, each T cell only expresses one type of T cell receptor.
Where do T cells develop?
T cell development begins in the bone marrow from a common lymphoid progenitor. The progeny that forms T cells leave the bone marrow and migrate to the thymus. Thus, giving it the term “thymus-dependent (T) cells“. Here they multiply and undergo a series of maturation steps. After that, they can undergo antigen-induced activation and differentiation into effector cells and memory cells.
The precise identity of the bone marrow cell precursor that gives rise to T cells is still debated. However, this precursor carries the potential to give rise to more than one type of cell. These cells being natural killer (NK) cells, dendritic cells (DC), B cells, and even myeloid cells.
What is the site of T cell maturation?
The T lymphocytes develop in the bone marrow but migrate to the thymus for maturation. During their maturation in the thymus, the T cells develop to express unique antigen-binding molecules, known as T cell receptors (TCR) on the membrane.
Role of the thymus in T cell maturation
The thymus is the major site of maturation of T cells. This function of the thymus was first suspected because of immunologic deficiencies associated with the lack of a thymus. The congenital absence of the thymus leads to a low number of mature T cells. In turn, it leads to severe deficiencies in T cell-mediated immunity. For example, DiGeorge’s syndrome in humans, and nude mutation in mice (which also causes hairlessness).
The rate of T cell development in the thymus is greatest before puberty. The thymus shrinks with age and is virtually undetectable in adult humans. This results in a somewhat reduced number of mature T cells. However, the maturation of T cells continues throughout adult life. All things considered, it may be that the remnant of the involuted (shrunk) thymus is adequate for some T cell maturation. Besides, it is known that memory T cells have a long life span (perhaps longer than 20 years in humans). So, the need to generate new T cells decreases as individuals age.
Key point: In both mice and humans, removal of the thymus after puberty is not accompanied by loss of T cell function. Thus, it seems that once the T cells are in enough numbers, immunity can be sustained without the production of new T cells.
Stages of T cell development
In this article, we divide T cell development stages as follows:
- Early Thymocyte Development
- Positive and Negative Selection
- Lineage Commitment
Early Thymocyte Development
Developing T cells in the thymus are called “thymocytes”. Developing thymocytes interact with the thymus stromal (non-haematopoietic) cells, and undergo the process described below in distinct regions of the thymus. The thymus consists of an outer cortex and an inner medulla. The cortex contains only immature thymocytes and scattered macrophages. Whereas, medulla contains mature thymocytes along with dendritic cells and macrophages.
Early thymocyte development is T cell receptor-independent. The specific events in the first phase (double-negative thymocyte development) include:
- The commitment of hematopoietic precursors to the T cell lineage,
- The initiation of receptor gene rearrangements, and
- The selection and expansion of cells that have successfully rearranged one of their T cell receptor genes (β selection).
The second phase is largely dependent on T cell receptor interactions. It brings cells to maturity from the CD4+ CD8+ (double positive, DP) stage to the CD4+ or CD8+ (single positive, SP) stage.
Key point: helper T cells and regulatory T cells express CD4 (specific for MHC class II). On the other hand, cytotoxic T cells express CD8 (specific for MHC class I).
The events in this last phase of development include:
- Positive selection: selection for those cells whose T cell receptors respond to self-MHC,
- Negative selection: selection against those cells whose T cell receptors react strongly to self-MHC/peptide combinations, and
- Lineage commitment: commitment of thymocytes to effector cell lineages, including CD4+ helper or CD8+ cytotoxic populations.
Where does T cell maturation occur?
After arriving in the thymus, T cell precursors encounter Notch ligands. They are abundantly expressed by the thymic epithelium. (We will read about this receptor later in this post)
In mice, it takes T cells 1 to 3 weeks to develop in the thymus. During this time, thymocytes pass through a series of stages defined by changes in their cell surface phenotype.
The subcapsular sinus and outer cortical region of the thymus contain the most immature thymocytes. From here, the thymocytes migrate into and through the cortex. This is where most of the subsequent maturation occurs. It is in the cortex that the thymocytes first express γδ and αβ TCRs. The αβ T cells mature into CD4+ class II MHC-restricted or CD8+ class I MHC-restricted T cells as they leave the cortex and enter the medulla. CD4+ and CD8+ single-positive (SP) thymocytes exit the thymus through the circulation from the medulla.
During T cell maturation, there is a precise order in which TCR genes are rearranged and in which the TCR and CD4 and CD8 coreceptors are expressed. In the mouse, surface expression of the γδ TCR occurs first, 3 to 4 days after precursor cells arrive in the thymus. Additionally, 2 or 3 days later the αβ TCR expression follows. In the human fetal thymus, γδ TCR expression begins at about 9 weeks of gestation, followed by expression of the αβ TCR at 10 weeks.
Key point: In mice, immature lymphocytes are first detected in the thymus on the 11th day of gestation. This corresponds to about week 7 or 8 of gestation in humans.
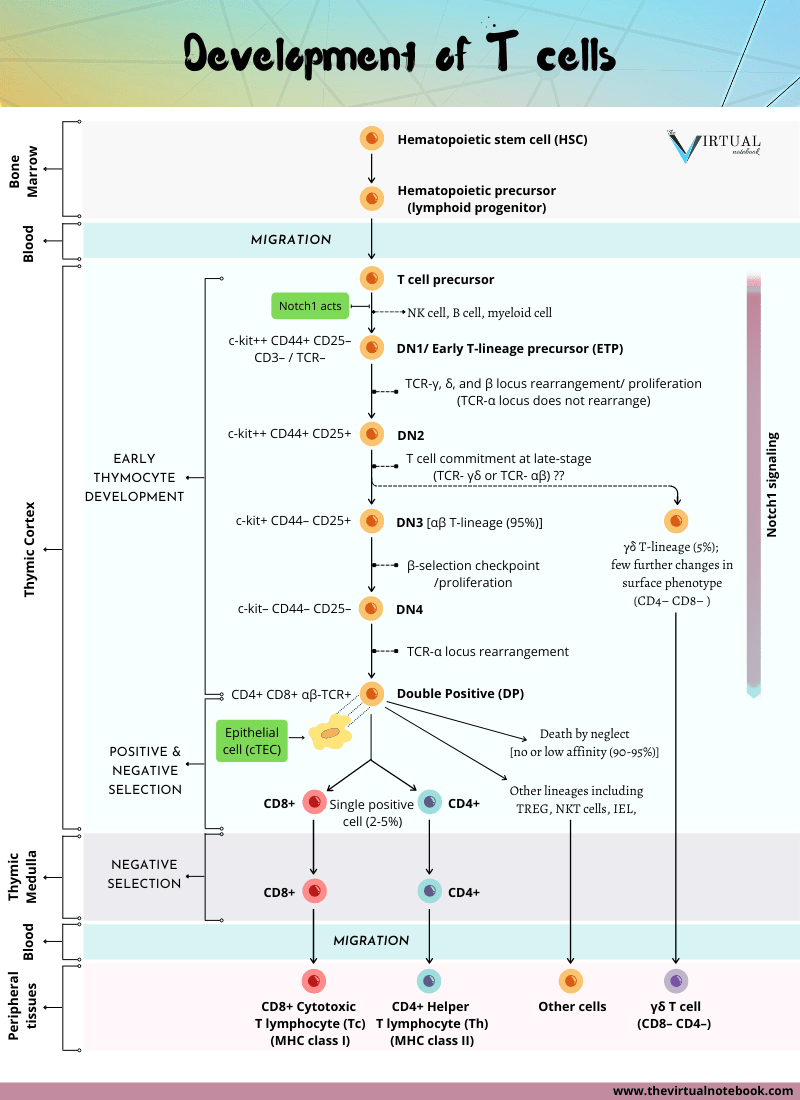
Double-negative thymocyte development
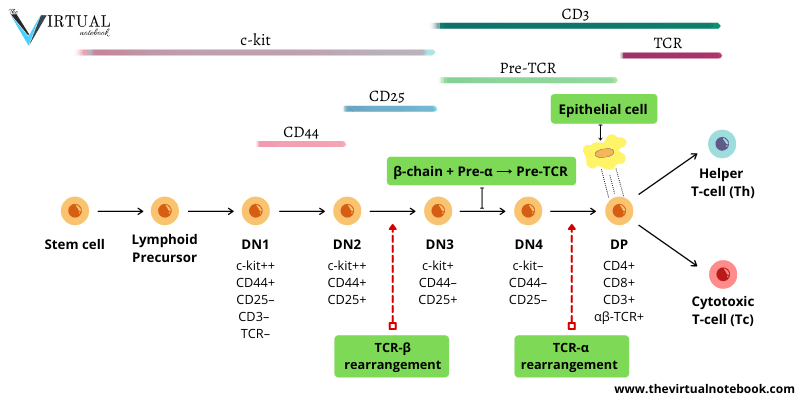
The earliest T cells lack CD4 and CD8. Hence, they are referred to as double-negative (DN) cells. These cells are also CD3/TCR negative, therefore can be termed triple-negative. DN T cells can be subdivided into four subsets: DN1–4. This is based on the presence or absence of other cell surface molecules, including c-kit (CD117), CD44, and CD25.
T cell development markers
There are two glycoproteins, CD4 and CD8. CD stands for cluster of differentiation. A protein with sugar attached to it is a glycoprotein. With their help, we can determine if a T cell is a helper T cell or a cytotoxic T cell.
There are a bunch of markers that help immunologists differentiate one cell from another. We often use the terms CD4+ or CD8+ when talking about a cell that expresses one of these markers. In T cell development, CD4 and CD8 are the most important markers. Other markers involved are:
- C-kit: the receptor for stem cell growth factor. It is expressed initially from the stem cell stage up until its maximal expression at the DN2 stage. By the time it reaches DN4, c-kit expression becomes too low.
- CD44: an adhesion molecule. It is only expressed in DN1 and DN2 stages.
- CD25: the α chain of the IL-2 receptor. It is expressed at the DN2 and DN3 stages.
Neither CD44 nor CD25 is expressed at the DN4 stage. This means that you can tell which stage it is in by knowing if the cell expresses any of the CD44, CD25, CD4 and/or CD8.
Other than CD4 and CD8, there are two very important markers, Pre-TCR and TCR. There are plenty of more markers that can further differentiate these cell types. We are going to talk about some of them as we progress through the stages.
Thymocytes progress through four double-negative (DN) stages
- DN1 thymocytes are the first to enter the thymus and are also termed early T lineage precursor (ETP). Despite that, they are capable of giving rise to multiple cell types. They express only c-kit and CD44 (c-kit++CD44+CD25–). But, once they enter the thymic environment and reach the cortex, they proliferate and express CD25. Then, they become DN2 thymocytes (c-kit++CD44+CD25+). (As shown in figure 1)
- During this stage of development (DN1–>DN2), the genes for the TCR-γ, δ, and β chains begin to rearrange. However, the TCR-α locus does not rearrange. Presumably, because the region of DNA containing TCR-α gene is not yet accessible to the recombinase machinery.
- At the late DN2 stage, T cell precursors fully commit to the T cell lineage. Also, they reduce the expression of both c-kit and CD44 (c-kit+CD44–CD25+).
- Cells in transition from the DN2–>DN3 stages continue rearrangement of the TCR-γ, δ, and β chains. At this point, they make their first major decision in T cell development. Whether to join the TCR- γδ or TCR- αβ. This choice is dictated by when and how fast the genes that code for each of the four receptor chains successfully rearrange.
- Hence, DN2 cells give rise to two T cell lineages. (a) The minority population of γδ T cells (which lack CD4 or CD8 even when mature), and (b) The majority of αβ T cell lineage (DN3).
- Now, the DN3 (c-kit+CD44–CD25+) T cells that commit to the TCR–αβ T cell lineage, undergo a process termed β-selection (described below). Later, these DN3 T cells lose expression of CD25 and halt proliferation. From here on, they enter the final phase of their DN stage of development (DN4).
- DN4 (c-kitlow-CD44–CD25–) matures directly into CD4+CD8+ DP (double-positive) thymocytes.
DN thymocytes undergo β-selection
β-selection is a process that selects for cells that have successfully rearranged their TCR-β chain locus (DN3 T cells). This process involves a uniquely expressed protein at this stage of development, a glycoprotein known as the pre-Tα chain. The β chains expressed by DN3 cells pair with this surrogate α chain. It allows them to assemble a pre-TCR that is analogous to pre-BCR (pre-B cell receptor) in structure and function. The pre-TCR is accompanied by the CD3 marker.
The assembly of the CD3/pre-TCR (DN3, DN4, DP) complex stimulates:
- Cell proliferation,
- The arrest of further β-chain gene rearrangements,
- The expression of CD8 and CD4, and
- Signals cells to act as a sensor for TCR-α chain locus rearrangement.
Double-positive (DP) thymocytes comprise the vast majority of thymocytes. Once the large double-positive thymocytes cease to proliferate and become small double-positive cells, the α-chain locus begins to rearrange. The structure of the α locus allows multiple successive rearrangement attempts. As a result, most developing thymocytes express a successful rearrangement at the α-chain locus. Thus, most DP cells produce αβ T cell receptor (αβ–TCR).
Key point: cells that fail to successfully rearrange the β locus remain in the CD44low CD25+ (DN3) stage and soon die. Whereas cells that make productive β-chain gene rearrangements and express the β chain, lose expression of CD25 once again.
Development of T cells in the mouse
Each stage of T cell development occurs in a specific microenvironment. Given that, they are characterized by specific intracellular events and distinctive cell-surface markers. The most immature thymocytes are CD4– CD8– (double negative, DN).
- At first, they pass through several stages (DN1–DN4).
- As shown in figure 1, they commit to the T cell lineage and rearrange their T cell receptor (TCR) gene loci. Those that successfully rearrange their TCR-β chain proliferate, initiate rearrangement of their TCR-α chains and become CD4+ CD8+ (double-positive, DP) thymocytes.
- At this point, DP thymocytes undergo negative and positive selection in the thymic cortex. Positively selected thymocytes continue to mature and migrate to the medulla.
- Thereafter, they go through negative selection to self-antigens.
- At last, Mature T cells express either CD4+ or CD8+ (single positive, SP). Then they leave the thymus with the potential to initiate an immune response.
- Although most thymocytes develop into conventional TCR-αβ CD4+ or CD8+ T cells, some DN and DP thymocyte cells develop into other cell lineages. They comprise lymphoid dendritic cells, TCR-γδ T cells, natural killer T cells (NKT), regulatory T cells (TREG), and intraepithelial lymphocytes (IELs). Each of which has a distinct function.
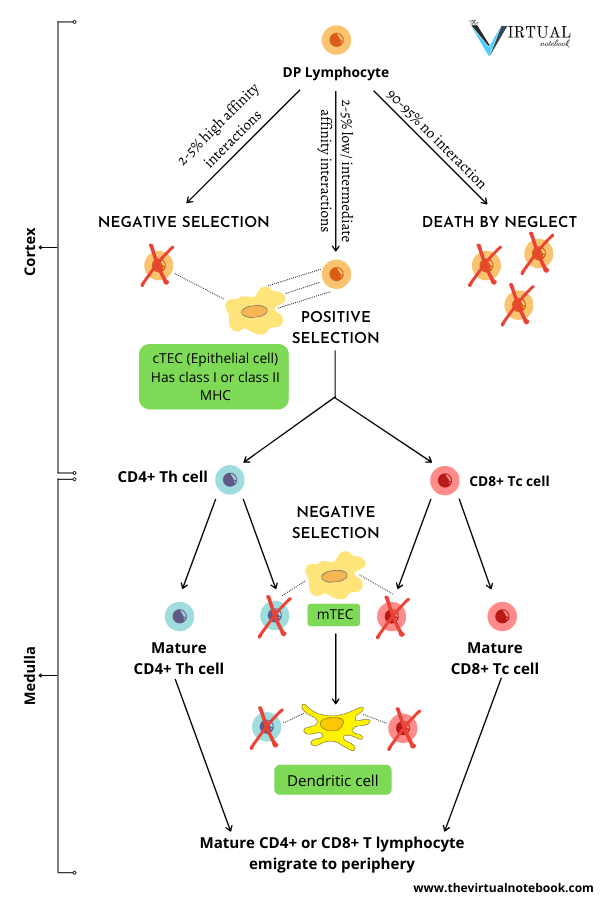
Positive and negative selection
Small, non-proliferating CD4+ CD8+ (DP) thymocytes that reside in the thymic cortex are the most abundant subpopulation in the thymus. They comprise more than 80% of cells. Initially, they express low levels of TCR. Most importantly, they are the first subpopulation of thymocytes that express a fully mature surface αβ-TCR/CD3 complex. Therefore, they are the primary targets of thymic selection. Thymic selection shapes the TCR repertoire of DP thymocytes. This is based on the affinity of their T cell receptors for the MHC/peptides.
Why is thymic selection necessary?
The most distinctive property of the mature T cells is that they recognize only foreign antigens combined with self-MHC molecules. The immature or unselected T lymphocytes may recognize any peptide antigen (self or foreign) displayed by any MHC molecule (also self or foreign). With that in mind, two distinct selection processes are required:
- Positive selection, and
- Negative selection.
DP thymocytes undergo positive and negative selection
Thymic selection involves multiple interactions of DP and SP thymocytes. This takes place with both the cortical and medullary thymic stromal cells and dendritic cells and macrophages. Selection results in a mature T cell population that is both self-MHC restricted and self-tolerant.
- DP thymocytes that express new TCR-αβ dimers browse the MHC/peptide complexes expressed by the “cortical thymic epithelial cells (cTECs)”.
- The majority of DP thymocytes bear receptors that cannot recognize (do not bind) self-MHC molecules. They fail positive selection and die (death by neglect).
- The small percentage whose TCRs bind MHC/peptide with high affinity die by clonal deletion.
- On the other hand, those double-positive cells that recognize self-MHC undergo positive selection. Later, they go on to mature and express high levels of the T cell receptor. Concurrently, they cease to express one or other of the two co-receptor molecules (CD4+ or CD8+). As a result, they become either CD4 or CD8 single-positive (SP) cells.
- SP thymocytes then migrate to the medulla. Here they come to meet “medullary thymic epithelial cells (mTECs)”.
- Medullary dendritic cells can acquire mTEC antigens by engulfing mTECs and mediate negative selection. Particularly of MHC Class II-restricted CD4+ thymocytes.
- Then, negative selection eliminates cells capable of responding to self-antigens. In every individual, T cells that recognize self-antigens with high affinity are potentially dangerous because such recognition may trigger autoimmunity.
Approximately 2-5% of the double positives survive this dual screening and mature as single-positive T cells. Then they leave the thymus and enter the peripheral circulation.
Key point: It is clear that thymocytes undergo positive selection in the cortex. Conversely, the medulla is the site of negative selection to tissue-specific antigens. Regardless, thymocytes undergo negative selection during and after the DP stage.
Fate of DP thymocytes
In the thymus, DP thymocytes come into contact with thymic epithelial cells. These cells express high levels of class I and class II MHC molecules on their surface. DP thymocytes undergo a selection process, depending on the signals they receive when they encounter self-MHC/self-peptide combinations with their TCRs. The strength of the encounter between TCRs and self-antigen–MHC complexes determine the outcome of this process.
Consequently, DP thymocytes can be considered to have one of three fates:
- The bulk of DP thymocyte death (~95%) occurs among thymocytes that fail positive selection. For the reason that their receptors do not bind to any of the MHC/self-peptides. These cells do not receive survival signals through their TCR and die by a process known as death by neglect (within 3 to 4 days).
- Negative selection: If they bind too strongly to MHC/self-peptide complexes they encounter (2-5%).
- Positive selection: If they bind with an intermediate affinity to MHC/self-peptide complexes. From here on, they go on to mature and become single-positive (SP) thymocytes. Only 2% to 5% of DP thymocytes exit the thymus as mature T cells. Without a doubt, it’s a process that induces both survival and differentiation of DP thymocytes.
Most developing T cells die in the thymus
T cell precursors arriving in the thymus from the bone marrow spend up to a week differentiating there before they enter a phase of intense proliferation. In a young adult mouse, the thymus contains around 108 to 2 × 108 thymocytes. About 5 × 107 new cells are generated each day. However, only about 106 to 2 × 106 (roughly 2–4%) of these will leave the thymus each day as mature T cells.
Despite the discrepancy between the numbers of T cells generated daily in the thymus and the number leaving, the thymus does not continue to grow in size or cell number. This is because approximately 95% of the thymocytes that develop in the thymus also die within the thymus. No widespread damage is seen, indicating that death is occurring by apoptosis rather than by necrosis.
Changes in the plasma membrane of cells undergoing apoptosis lead to their rapid phagocytosis. In turn, this shows a build-up of Apoptotic bodies (residual condensed chromatin of apoptotic cells) inside macrophages throughout the thymic cortex. This decay of thymocytes is a crucial part of T cell development. It reflects the intensive screening each new thymocyte undergoes for the ability to recognize self-MHC and for self-tolerance.
Surely, the rate of cell proliferation and apoptotic death is extremely high in cortical thymocytes. Cell death is due to a combination of factors. These factors include:
- A failure to productively rearrange the TCR-β chain and thus, to negotiate the pre-TCR/β selection checkpoint,
- A failure to be positively selected by MHC molecules in the thymus, resulting in self-antigen–induced negative selection.
- Cortical thymocytes are also sensitive to irradiation and glucocorticoids. In vivo, high doses of glucocorticoids induce apoptotic death of immature cortical thymocytes.
Lineage commitment
As thymocytes are being screened based on their TCR affinity for self-antigens, they are also being guided in their lineage decisions. Specifically, a positively selected double-positive (DP) thymocyte must decide whether to join the CD8+ cytotoxic T cell lineage or the CD4+ helper T cell lineage. Lineage commitment requires changes in genomic organization and gene expression that results in:
- Silencing of one co-receptor gene (CD4 or CD8), as well as
- Expression of genes associated with a specific lineage function.
But, what is responsible for this decision?
The importance of Notch in T cell commitment
Commitment to the T cell lineage is dependent on a receptor called “Notch”. Additionally, it is linked with embryonic cell development. Notch controls the decision of a lymphoid precursor in becoming a T versus a B lymphocyte.
Out of four known genes that form the Notch family of protein receptors, there is one active version, named Notch1. Now the question is, how does this Notch1 receptor influences the fate of lymphocytes?
When Notch1 is overexpressed in stem cells, T cells rather than B cells develop in the bone marrow. Reciprocally, when the Notch1 gene is knocked out of hematopoietic precursors, B cells rather than T cells develop in the thymus.
Double-positive thymocytes may commit to other types of lymphocytes
Small populations of DP thymocytes can also commit to other T cell types. They include:
- NKT cells (natural killer T cells), which includes mature cells that express only CD4 and cells that have lost both CD4 and CD8. They play a role in innate immunity and express a TCR that includes an invariant TCR-α chain. For this reason, NKT cells are sometimes referred to as iNKT cells. Their invariant TCR does not interact with classical MHC, but with the related molecule CD1, which presents glycolipids and not peptides.
- Intraepithelial lymphocytes (IEL), most of which are CD8+, also have features of innate immune cells and patrol mucosal surfaces.
- Regulatory T cells (TREG), another CD4+ subset, quench adaptive immune reactions.
All three subpopulations are thought to develop from DP thymocytes in response to autoreactive, high-affinity TCR interactions. The same interactions mediate negative selection.
Sources
Abul K. Abbas, Andrew H. Lichtman and Shiv Pillai. (2012). “Lymphocyte Development and Antigen Receptor Gene Rearrangement.” Cellular and molecular immunology. 7th edition. 194–202.
Charles A. Janeway Jr., Paul Travers, Mark Walport and Mark J. Shlomchik. (2001) “Generation of lymphocytes in bone marrow and thymus.” Immunobiology: The Immune System in Health and Disease. 5th edition.
Gustavo Glusman, Lee Rowen, Inyoul Lee, et al. (2001). “Comparative Genomics of the Human and Mouse T Cell Receptor Loci”. 15(3), 337-349. Immunity. DOI:https://doi.org/10.1016/S1074-7613(01)00200-X
Judith A. Owen, Jenni Punt and Sharon A. Stranford. (2013) “T cell development”. Kuby immunology. 7th edition. 299-354.