The term complement refers to a set of serum proteins that eliminate blood and tissue pathogens. The complement system exists in an inactive state in the plasma. Moreover, it is an extremely powerful system comprising of glycoproteins, several proenzymes, and components. All normal individuals always have complement components in their blood that exist in a non-active state in the serum. In addition, the complement system consists of approximately 20 proteins that are present in normal human serum. When these complement components convert to their active form, a sequential, rapid, cascading sequence occurs.
The primary function of the complement system is to bind and neutralize any foreign substance that activates it. Another function of the complement system is to enhance phagocytosis.
HISTORY
In the 1890s, Jules Bordet showed that sheep antiserum to the bacterium Vibrio
This finding led Bordet to reason that bacteriolysis required two different substances:
- the heat-stable specific antibodies that bound to the bacterial surface,
- heat-labile (sensitive) component responsible for the lytic activity.
The heat-stable specific antibody was responsible for immunity against specific microorganisms, whereas the heat-sensitive component was responsible for the non-specific antimicrobial activity.
In 1899, Paul Ehrlich carried out similar experiments and coined the term ‘complement’, defining it as “the activity of blood serum that completes the action of antibody.”
PROPERTIES OF COMPLEMENT SYSTEM
Complement shows the following properties:
- It is present in sera of all mammals including humans and in lower animals including birds, amphibians, and fishes.
- These are heat-labile substances that can be inactivated by heating serum at 56°C for 30 minutes.
- Most complement components synthesized in the liver by hepatocytes, although some will also be produced by other cell types. These cell types include blood monocytes, tissue macrophages, fibroblasts, and epithelial cells of the gastrointestinal and genitourinary tracts.
- The complement usually does not bind to the antigen or antibody but only to antigen-antibody complex.
- The importance of the complement lies in the fact that it contributes to both the acquired and innate immunity of an individual.
- The synthetic rates for the various complement glycoproteins increase when complement will activate and consume during an infection.
ACTIVATION OF COMPLEMENT SYSTEM
Complement activation takes place through any of the following three pathways:
- The classical pathway
- The alternative pathway
- The lectin pathway
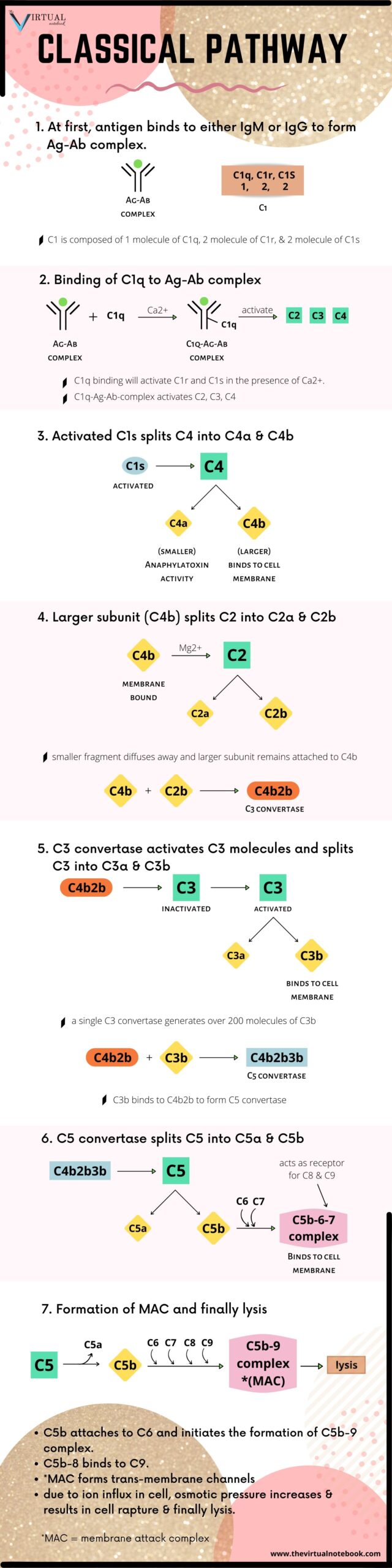
1. The classical pathway
The classical pathway is a chain of events in which complement components react in specific sequences as a cascade resulting in cell lysis
Steps in the activation of classical pathways are:
Activation of C1
Native, free immunoglobulin does not activate the complement system. A single native IgG molecule will not bind and activate the first component (C1) in the complement pathway.
for antibody structure, see: Immunoglobulin structure
C1 is actually a complex of three different types of molecules (one C1q molecule, two C1r molecules, and two C1s molecules) held loosely together through non-covalent bonds and requiring a physiological Ca2+ concentration for their proper association. C1q first combines with the Fc portion of the bound antibody, IgM or IgG. This results in the sequential activation of C4, C2, and C3.
When pentameric IgM bound to an antigen on a target surface, it assumes the stable configuration. Since IgG molecules have a lower valency, about 1000 of them will require to ensure the initiation of the complement pathway as against only one IgM molecule.
Activation of C1r and C1s
C1q binding in the presence of calcium ions leads to activation of C1r and C1s. Activated C1s splits C4 into two fragments:
- a small soluble fragment (C4a): it has anaphylatoxin activity.
- a larger fragment (C4b): it binds to cell membrane along with C1 and splits C2 into C2a and C2b in the presence of Mg2+.
The smaller fragment (C2a) diffuses away, while the larger fragment (C2b) remains attached to C4b. The resulting C4b2b complex(C3 convertase) possesses enzymatic activity, which converts C3 into an active form.
(Important tip: Please note that in some books [and online places too] C2a refers as the larger subunit and not the smaller subunit. Wikipedia also clears it too that historically, the larger fragment of C2 was called C2a but is now referred to as C2b.) If you will write about the complement system in your exam, I suggest you be clear at the beginning about your larger and smaller subunit symbols and make sure you underlined it. I am saying you to underlined it because underlined words grab faster attention of examiner so that he/she won’t miss your point. If you don’t, chances are high that you will lose your well-deserving marks and trust me… you won’t be happy with that… So be extra cautious)
Role of C3 convertase
The C3 convertase activates thousands of C3 molecules and splits these molecules into C3a and C3b. Moreover, a single C3 convertase molecule can generate over 200 molecules of C3b, resulting in tremendous amplification of the sequence.
Role of C5 convertase
Some of the C3b binds to C4b2b to form a trimolecular complex C4b2b3b called C5 convertase. The C5 convertase splits C5 into C5a and C5b. Unlike other complement fragments, C5b does not bind immediately to the nearest cell membrane. A complex of C5b, C6, and C7 is first formed, and then the C5b67 complex attaches to the cell membrane. The membrane-bound C5b-6-7 complex acts as a receptor for C8 and then C9. Meanwhile, C8 binds to the complex and stabilize the attachment of the complex to the foreign cell membrane. After that, C5b attaches to C6 and initiates the formation of C5b–9 complex otherwise known as membrane attack complex (MAC).
The C5b–8 complex on binding to C9 molecules undergoes polymerization, which finally ends in the formation of C5b–9 complex also known as MAC. The MAC forms a transmembrane channel that allows the free exchange of ions between the cell and the surrounding medium. Due to the rapid influx of ions into the cell and their association with cytoplasmic proteins, the osmotic pressure rapidly increases inside the cell. This results in an influx of water, swelling of the cell, and, for certain cell types, rupture of the cell membrane and finally lysis.
I tried to write the classical pathway into as simple steps as I can and create an infographic below… please go through it.
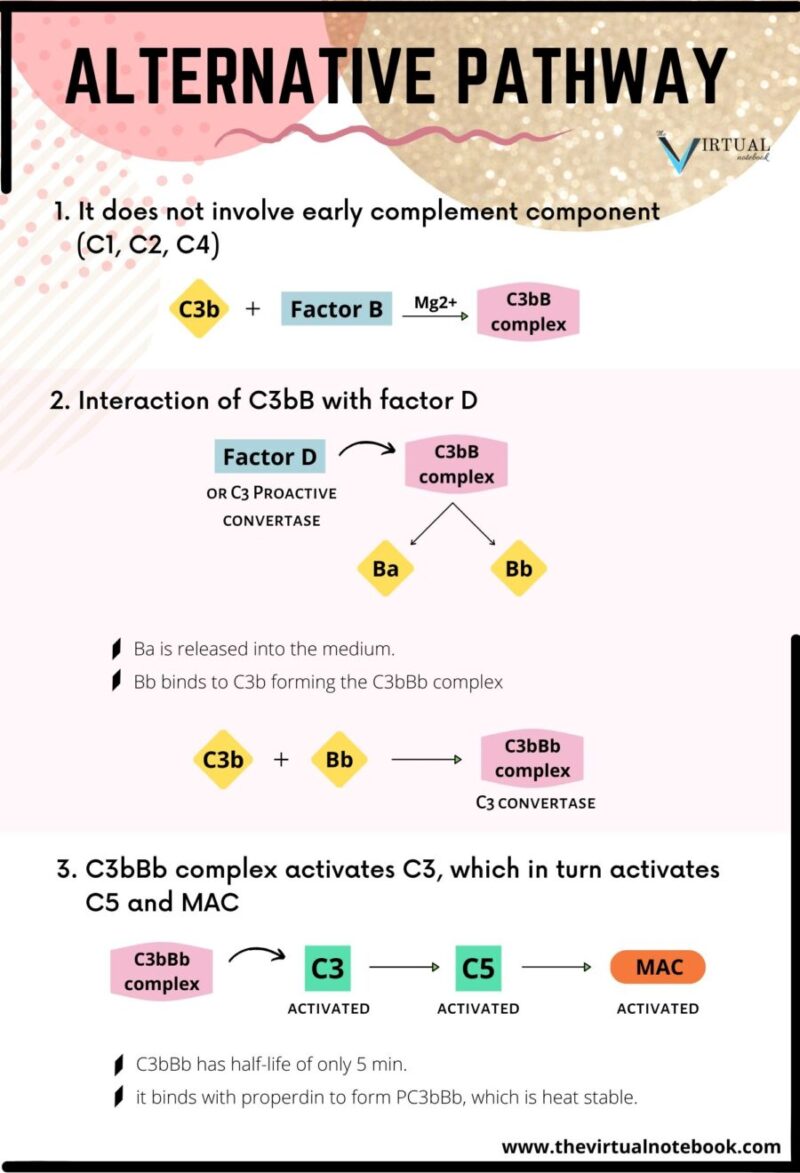
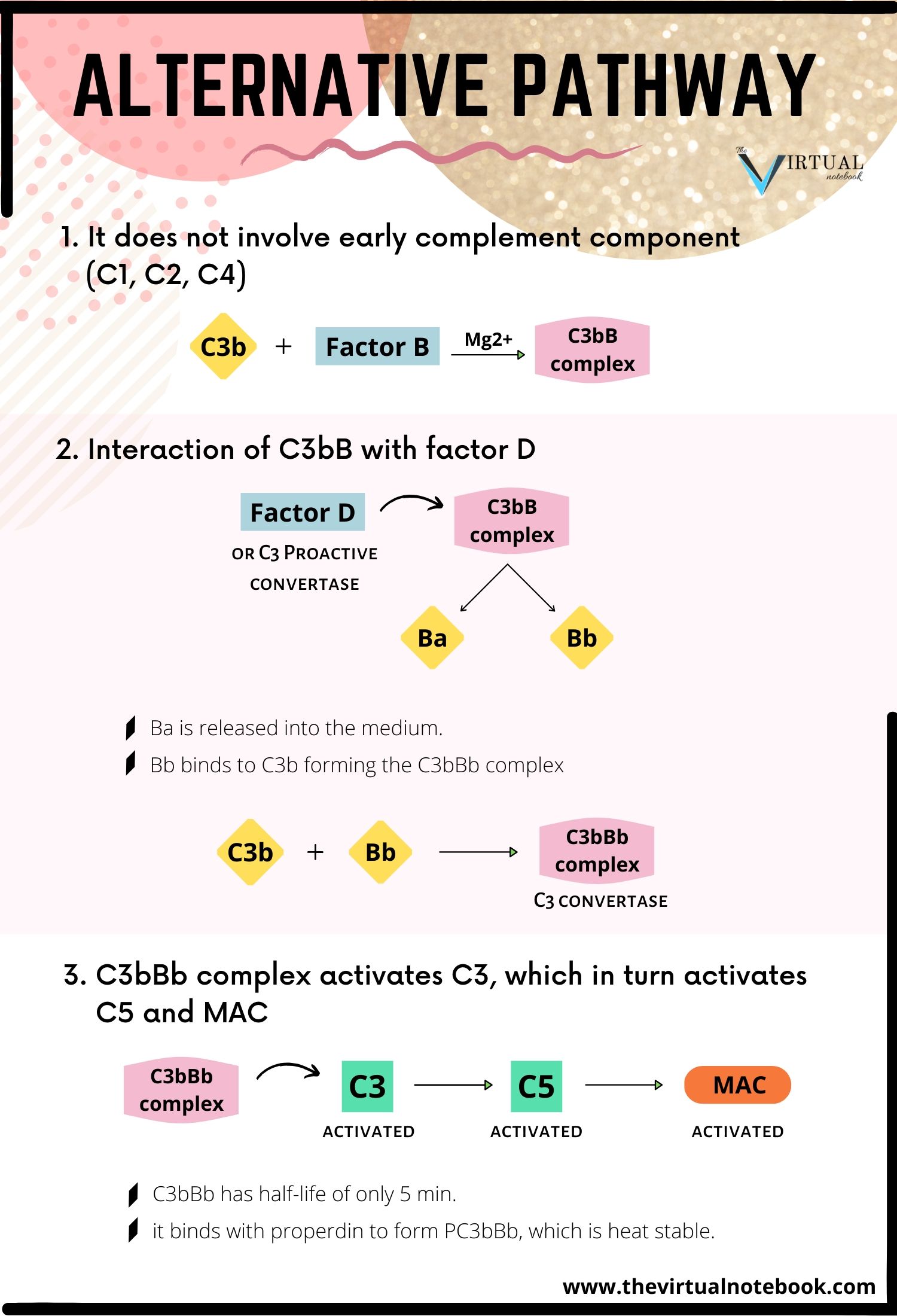
2. The alternative pathway
The alternative pathway was first described by Pillemer in 1954. Initiation of the alternative pathway of complement activation is independent of antibody-antigen interactions. The alternative pathway is unique as it can be activated before the establishment of an immune response to the infecting pathogen.
Activators of alternative pathways:
- lipopolysaccharides (bacterial membrane) of gram-negative bacteria.
- peptidoglycans and teichoic acids from the cell walls of certain gram-positive bacteria.
How it differs from the classical pathway?
- the pathway is different in the nature of activating substances, and
- unlike classical pathway, it does not depend on antibody and does not involve early complement components (C1, C2, C4) for activation of the complement.
Steps in the activation of alternative pathways are:
Formation of C3bB complex
The C3b binds with factor B to form C3bB complex. The interaction between C3b and factor B is stabilized by Mg2+, which is the only ion required for functional activation of the alternative pathway.
Interaction of C3bB with factor D
The C3bB is split into two fragments, Ba and Bb, by another serum protein, factor D or C3 proactive convertase. Factor D is synthesized in hepatocytes. Since factor D has never been isolated in its proenzyme form, it is generally activated immediately upon leaving the hepatocyte. After that, Ba is released into the medium and the Bb binds to C3b forming the C3bBb complex. This complex possesses C3 convertase activity.
Activation of C5 and MAC
The C3bBb complex activates more C3, leading to the formation of more C3bBb, which in turn is capable of activating C5 and the MAC. The C3bBb complex has a half-life of only 5 minutes, but by binding with properdin it forms PC3bBb complex, which is relatively heat-stable.
Production of MAC
The alternative pathway then proceeds from C3 to produce finally the MAC, in the same way as occurs in the classical pathway.
The biological significance of the alternative pathway: it is important, especially during the early phase of the infection when the concentrations of a specific antibody are very low.
Lectin pathway
The lectin pathway is triggered by lectins. Lectins are the proteins that recognize and bind to specific carbohydrate targets. The pathway involves the serum mannan-binding lectin(MBL), sometimes termed mannose-binding lectin. MBL is an acute phase reactant, meaning that its concentration increases during infection/inflammation.
The first step in the lectin pathway requires the direct binding of serum mannan-binding lectin to polysaccharides (e.g., mannan) on the surface of microorganisms. In addition to carbohydrate motifs of microorganisms, MBL can bind to glycoproteins on the envelope of certain viruses such as influenza A.
The lectin complement pathway is initiated by microorganism-bound MBL as it associates with two human MBL-associated serine proteases (MASP-1 and -2) that cleave and activate C4 and C2. MASP-1 and MASP-2 are similar to C1r and C1s, respectively, and MBL is similar to C1q. In addition, the MBL complex generates deposition of C4b2a with subsequent C3b deposition and terminal component activation. Subsequently, it proceeds to produce MAC in the same way as that occurs in the classical and alternative pathways.
It is important to realize that classical pathway activation by immune complexes is much more efficient and powerful than activation by the lectin pathway.
REGULATION OF COMPLEMENT SYSTEM
The complement system has the potential to be extremely damaging to host tissues, meaning its activation must be tightly regulated. The complement system is regulated by complement control proteins, which are present at a higher concentration in the blood plasma than the complement proteins themselves. There are many regulatory systems to prevent unwarranted damage to the human host. The following are regulators of the complement system:
Level of antibody
It is the first regulatory step in the classical pathway. If an antigen is not bound to the antibodies, the complement-binding sites are unavailable to the C1 component of the complement. This means that complement is not activated even if IgM and IgG are present in the blood at all times. However, when antigen binds with specific antibodies, a conformational shift occurs and that allows the C1 component to bind and initiate the cascade reaction.
C1 inhibitors
They prevent the formation and function of C1qrs complex by causing C1s to dissociate from C1qrs. The C1 inhibitors may also aid in the removal of the entire C1 complex from the antigen–antibody complexes.
Other inhibitory substances
Multiple substances have inhibitory effects over different steps of the activation sequence of the classical pathway. These mechanisms probably help to protect the host cells from the possible bystander damage initiated by activated complement fragments (C3b and C4b).
Decay-accelerating factor (DAF)
It is another inhibitory substance that can accelerate the dissociation of active C4b2a complexes. It inhibits the activity of C3 convertase. In addition, DAF remains attached to membrane-bound C4b and C3b and prevents the subsequent interaction with C2a and factor B, respectively.
Regulation of alternative pathway
The alternative pathway has its own set of regulatory proteins and mechanisms. It is mediated by the binding of factor H to C3b and cleavage of this complex-by specific plasma inhibitor factor I, a protease. This reduces the amount of C5 convertase available.
DEFICIENCY/DISEASE OF COMPLEMENT
Deficiency of various components may result in many diseases as follows:
- The low level of C1 esterase inhibitors leads to overproduction of esterase. This leads to an increase in the release of anaphylatoxins, which cause capillary permeability and
edema . - Acquired deficiency of DAF results in an increase in complement-mediated hemolysis.
- Inherited or acquired deficiency of C5–8 components greatly enhances susceptibility to Neisseria bacteremia and other infections. Deficiency of C3 leads to severe recurrent pyogenic sinusitis and respiratory infections.
- A deficiency in MBL, the first component of the lectin pathway, is relatively common and results in serious pyrogenic (fever-inducing) infections in babies and children. Children with MBL deficiency suffer from respiratory tract infections.
- People with C3 deficiencies display the most severe clinical manifestations of any of the complement deficiency patients, reflecting the central role of C3 in opsonization and in the formation of the MAC.
REFERENCES
A textbook of Microbiology and Immunology, 2nd edition by Subhash Chandra Parija, chapter 15: Complement System
Kuby immunology, 7th edition, chapter no. 6: The complement system
Medical immunology, fifth edition revised and expanded, edited by Gabriel Virella, part 1: Basic immunology, chapter no. 9: The complement system
Thanks for the easier discussion on the comparatively difficult to grasp topic.
I am more than happy to help you