ANTIBODIES
Antibodies are globulin proteins (immunoglobulins) that will produce in response to antigens. Immunoglobulins are also known as antibodies. They are incredibly diverse and specific in their ability to recognize foreign molecular structures. Basically, B lymphocytes are the only cells that synthesize antibody molecules. In addition, antibodies make up about 20% of the protein in blood plasma. Overall, there are three types of globulins present in the blood namely, alpha, beta, and gamma. The antibodies are the gamma globulins.
The World Health Organization (WHO) in 1964 coined the term “immunoglobulin (Ig)” for the term antibody. The immunoglobulin includes not only antibody globulins but also the cryoglobulins, macroglobulins, and abnormal myeloma proteins. Thus, all antibodies are immunoglobulins but not all immunoglobulins may be antibodies.
Functions
The most important function of antibodies is to provide protection against microbial pathogens. Some of the main functions of antibodies are:
- they reduce the virulence of microbes by neutralizing toxins and viruses.
- they opsonize microbes so they are more easily phagocytosed,
- also, they activate the complement,
- antibodies will prevent the attachment of microbes to mucosal surfaces.
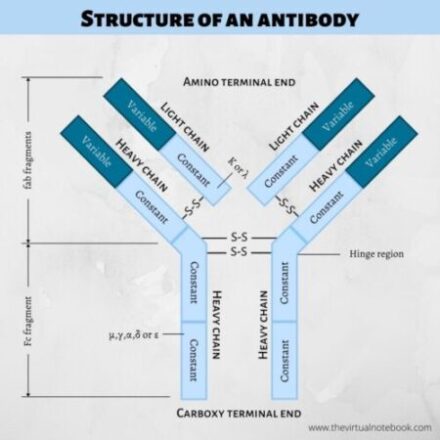
STRUCTURE OF ANTIBODIES/IMMUNOGLOBULINS
Immunoglobulins are glycoproteins comprises of four polypeptide chain: two identical light (L) and two identical heavy (H) chains. Further, L and H chains are subdivided into variable and constant regions. The terms light and heavy refer to molecular weight. The heavy chains are longer whereas light chains are shorter. Light chains have a molecular weight of about 25,000 Da whereas heavy chains have a molecular weight of 50-70,000 Da.
The simplest antibody molecule has a ‘Y’ or ‘T’ shape structure which is the most widely recognizable feature of immunoglobulin structure. All antibody molecules share the same basic structural characteristics but display remarkable variability in the regions that bind antigens. Because the core structural unit of each antibody molecule contains two heavy chains and two light chains, every antibody molecule has at least two antigen-binding sites.
Immunoglobulin (Ig) domain
Both the light chains and the heavy chains contain a series of repeating, homologous units. Each unit is about 110 amino acid residues long, that fold independently in a globular motif that is called an Ig domain. An Ig domain contains two layers of a β-pleated sheet, each layer composed of three to five strands of the antiparallel polypeptide chain. However, the number of strands per sheet varies among individual proteins.
Within the strands, hydrophobic and hydrophilic amino acids alternate and their side chains are oriented perpendicular to the plane of the sheet.
Heavy chains of immunoglobulins
An immunoglobulin molecule has two heavy chains. Each heavy chain comprises 420–440 amino acids. Also, each heavy chain binds to a light chain by a disulfide bond and by noncovalent bonds.
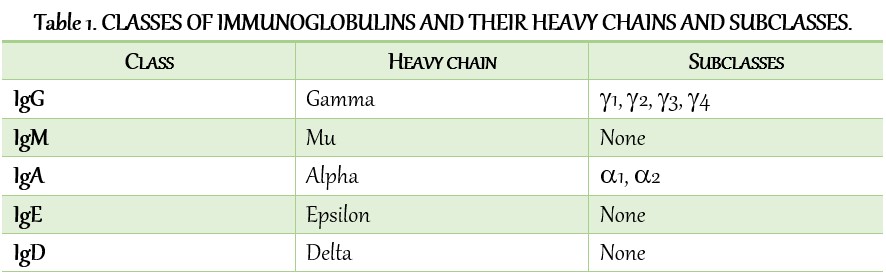
The sequences of the heavy-chain constant regions fall into five basic patterns. These five basic sequences named with Greek letters and these are:
- µ (mu)
- δ (delta)
- γ (gamma)
- ε (epsilon)
- α(alpha).
The heavy chains of a given antibody molecule determine the class of that antibody. For example, IgM contains µ (mu), IgG contains γ (gamma), IgA contains α(alpha), IgD contains δ (delta), and IgE contains ε (epsilon). heavy chains. These heavy chains are structurally and antigenically distinct for each class of immunoglobulin.
In addition, heavy chains exist in two forms that differ at their carboxyl-terminal ends. One form of the heavy chain anchors membrane-bound antibodies in the plasma membranes of B lymphocytes whereas the other
form is secreted when associated with Ig light chains.
Light chain
An immunoglobulin molecule has two light chains. Each light chain comprises 220–240 amino acids. Further, the light chain attaches to the heavy chain by a disulfide bond. Unlike heavy chains, the light chains are structurally and chemically similar in all classes of immunoglobulins. They are of two types:
- Κ (kappa), and
- λ (lambda).
Each immunoglobulin has either two Κ (kappa) or two λ (lambda) chains but never both. In humans, about 60% of antibody molecules have Κ (kappa) light chains and about 40% have λ light chains. The Κ (kappa) and λ (lambda) chains are present in human serum in a ratio of 2:1.
Each light chain comprises of one V (variable) region Ig domain and one C (constant) region Ig domain. Variable regions distinguish the antibodies made by one clone of B cells from the antibodies made by other clones.
Variable and constant region
Each polypeptide chain of an immunoglobulin molecule contains an amino-terminal part and a carboxy-terminal part. The amino terminal part is called the variable region (V region) whereas the carboxy-terminal part is called the constant region (C region).
The variable regions of both the light and heavy chain are responsible for antigen binding whereas the constant region of the heavy chain is responsible for various biologic functions. For example, complement activation and binding to cell surface receptors.
Constant region
The constant (C) region is the carboxyl-terminal of the molecule. It consists of two basic amino acid sequences. The constant region of the light chain does not have any biological function whereas the constant region of the heavy chain is responsible for activation of the complement, binding to cell surface receptors, placental transfer, and many other biological activities.
Moreover, the C region domains are separate from the antigen-binding site and do not participate in antigen recognition.
Variable region
The variable region consists of 100-110 amino acids at amino-terminal end. This region is different for each class of immunoglobulins. Basically, the variable regions of both L and H chains have three extremely variable (hypervariable) amino acid sequences at the amino-terminal end that form the antigen-binding site.
The hypervariable loops are like fingers protruding from each variable domain, three fingers from the heavy chain and three fingers from the light chain coming together to form an antigen-binding site.
These antigen-binding sites are responsible for specific binding of antibodies with antigens.
Fab fragments (fragment antigen-binding)
It is a region on an antibody that binds to antigens. It comprises one constant and one variable domain of each of the heavy and the light chain. These domains shape the antigen-binding site, at the amino terminal end of the monomer.
Fc fragments (fragment crystallizable)
Fc region is the tail region of an antibody that interacts with cell surface receptors called Fc receptors and some proteins of the complement system. This property allows antibodies to activate the immune system.
How many types of antibodies are there?
Antibodies, also known as immunoglobulins, are of five types.
- IgG
- IgA
- IgM
- IgD
- IgE
Lets discuss these types/classes one by one…
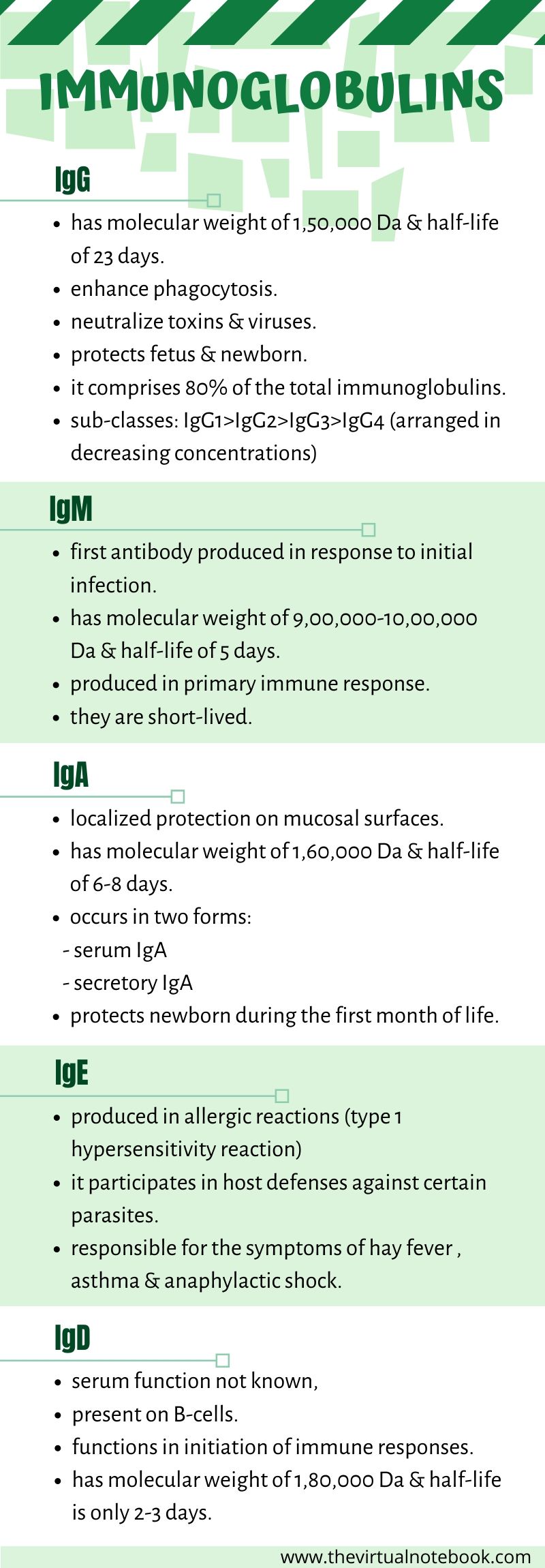
IgG
Each IgG molecule consists of two L chains and two H chains linked by disulfide bonds. It has a molecular weight of 1,50,000 Da and has a half-life of 23 days (longest among all the immunoglobulins). It is a divalent molecule because it has two identical antigen-binding sites.
IgG is the most abundant class of immunoglobulins in the serum, comprising about 80% of the total serum immunoglobulin. Depending upon antigenic differences in the H chains and on the number and location of disulfide bonds, there are four subclasses of IgG, namely: IgG1, IgG2, IgG3, and IgG4. They are numbered according to their decreasing concentrations in serum.
Chiefly, IgG1 makes up most (65%) of the total IgG. IgG2 antibody is directed against polysaccharide antigens and is an important host defence against encapsulated bacteria.
IgG is the only antibody to cross the placenta and is, therefore, the most abundant immunoglobulin in newborns. This is an example of passive immunity because the IgG is produced by the mother, not by the fetus.
to know more about passive immunity…… check notes on types of immunity
Functions
- IgG1, IgG3, and IgG4 are the only immunoglobulins with the ability to cross the placental barrier. Therefore, they play an important role in protecting the developing fetus against infections.
- IgG3, IgG1, and IgG2, in order of their efficiency, are effective in the activation of the complement.
- It takes part in precipitation, complement fixation, and neutralization of toxins and viruses.
- It binds to microorganisms and facilitates the process of phagocytosis of microorganisms.
IgA
IgA is the second major serum immunoglobulin, comprising nearly 10–15% of serum immunoglobulin. It has a half-life of 6–8 days. In addition, it is the main immunoglobulin in secretions such as colostrum, saliva, tears, and respiratory, intestinal, and genital tract secretions. Moreover, IgA occurs in two forms:
- serum IgA: It is present in the serum and is a monomeric molecule with a molecular weight of 60,000 Da. It has two subclasses, IgA1 and IgA2.
- secretory IgA: It is a dimer or tetramer and consists of a J-chain polypeptide. Secretory IgA is the major immunoglobulin present in external secretions, such as breast milk, saliva, tears, and mucus of the bronchial, genitourinary, and digestive tracts.
Functions
- It protects the mucous membranes against microbial pathogens. Because it is polymeric, secretory IgA can cross-link large antigens with multiple epitopes.
- Secretory IgA protects the newborns against infection during the first month of life. Because the immune system of infants is not fully functional, breastfeeding plays an important role in maintaining the health of newborns.
- Secretory IgA has shown to provide an important line of defence against bacteria and viruses.
IgM
IgM constitutes about 5–8% of total serum immunoglobulins. It is a heavy molecule with a molecular weight varying from 900,000 to 1,000,000 Da. It has a half-life of 5 days. IgM antibodies are short-lived and disappear early as compared to IgG. The presence of IgM antibody in serum, therefore, indicates recent infection.
IgM is the main immunoglobulin that produces early in the primary response. It is present as a monomer on the surface of virtually all B cells while in serum, it is present as a pentamer, composed of five immunoglobulin subunits and one molecule of J chain. Comparatively, it is more efficient than IgG in activating complement.
Because the pentamer has 10 antigen-binding sites, it is the most efficient immunoglobulin in agglutination, complement fixation (activation), and other antibody reactions.
Functions
- IgM confers protection against invasion of blood by microbial pathogens. Deficiency of IgM antibodies is associated with septicemia.
- IgM is not transported across the placenta; hence, the presence of IgM in the fetus or newborn indicates intrauterine infection. The detection of IgM antibodies in serum, therefore, is useful for the diagnosis of congenital infections, such as syphilis, rubella, toxoplasmosis, etc.

IgD
IgD comprises less than 1% of serum immunoglobulins. It is a monomer with a molecular weight of 180,000 Da. The half-life of IgD is only 2–3 days. it is present on the surface of many B lymphocytes and in small amounts in serum. B
Functions
- it initiates immune responses. However, its exact function is not known.
IgE
IgE constitutes less than 1% of the total immunoglobulin pool. It has a molecular weight of 1,90,000 Da and half-life of 2–3 days. Unlike other immunoglobulins that are heat stable, IgE is a heat-labile protein and it easily inactivate at 56°C in 1 hour.
It is present in serum in a very low concentration(0.002%). It is mostly present in the lining of the respiratory and intestinal tracts. Although, IgE is present in trace amounts in normal serum, persons with allergic reactivity have greatly increased amounts of IgE and it may appear in external secretions.
Moreover, IgE does not fix complement and does not cross the placenta.
Functions
- it mediates immediate (anaphylactic) hypersensitivity ( to know more about hypersensitivity, click on this link )
- it participates in host defenses against certain parasites.
SOURCES AND LINKS
Textbook of microbiology and immunology, 2nd edition, Subhash Chandra Parija, section II, chapter no. 13
Review of Medical Microbiology and Immunology by Warren Levinson, chapter no. 59
Kuby immunology, 7th edition, the structure of antibodies.
Cellular and Molecular Biology by Abul K. Abbas, chapter no. 5- antigens and antibodies.